New Arrival China SARS-CoV-2 RT-PCR - COVID-19 IgM/IgG Lateral Flow Assay – Genobio
New Arrival China SARS-CoV-2 RT-PCR - COVID-19 IgM/IgG Lateral Flow Assay – Genobio Detail:
Product Introduction
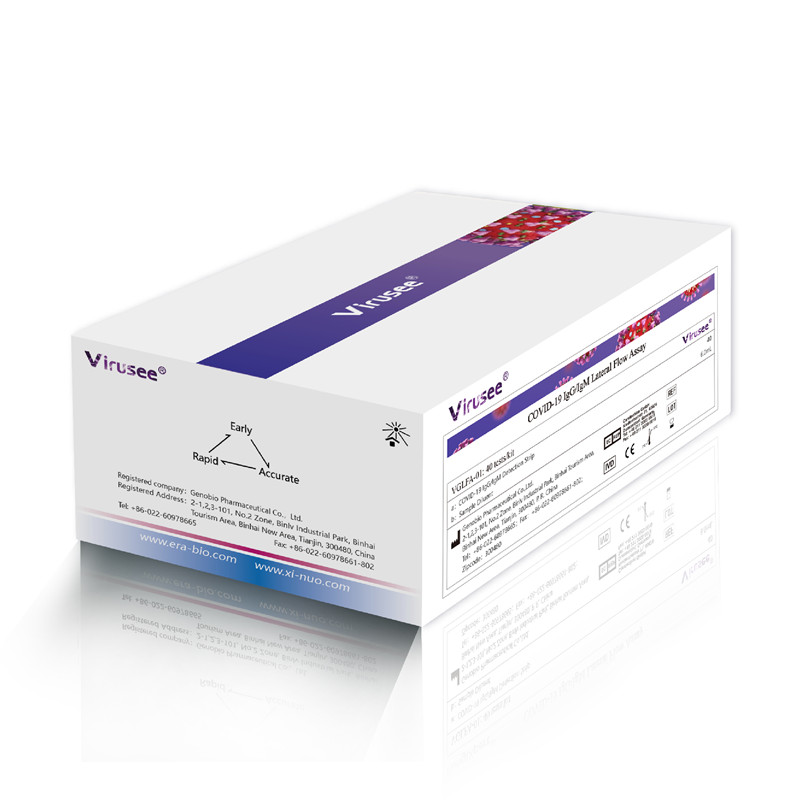
Virusee® COVID-19 IgM/IgG Lateral Flow Assay is a lateral flow immunoassay used for in vitro qualitative detection of novel coronavirus (SARS-CoV-2) IgM / IgG antibodies in human venipuncture whole blood, plasma, and serum specimens.
The novel coronavirus is a positive single-stranded RNA virus. Unlike any known coronavirus, the vulnerable population for Novel Coronavirus is generally susceptible, and it is more threatening to the elderly or people with fundamental diseases. IgM/IgG antibodies positive is an important indicator of novel coronavirus infections. Detection of novel coronavirus-specific antibodies will aid clinical diagnosis.
Characteristics
|
Name |
COVID-19 IgM/IgG Lateral Flow Assay |
|
Method |
Lateral Flow Assay |
|
Sample type |
Blood, plasma, serum |
|
Specification |
20 tests/kit |
|
Detection time |
10 min |
|
Detection objects |
COVID-19 |
|
Stability |
The kit is stable for 1 year at 2-30°C |

Advantage
- Rapid
Obtain result within 10 min - Simple
Visually reading result, easy to interpret
Simple procedure, without complicated operation
- Cost-saving
Product can be transported and stored at room temperature, reducing costs - Low risk
Testing blood sample, reducing the risk of sampling process - Suitable for screening on-site, bedside, outpatient
Background and principle
The novel coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV)-2, has been identified as the causative pathogen of coronavirus disease 2019 (COVID-19). This disease has been called a public health emergency of international concern by the World Health Organization (WHO).
COVID-19 targets the upper and lower respiratory systems and causes flu-like symptoms in most infected people. Although many COVID-19 patients experience only mild symptoms, some patients have severe symptoms leading to massive lung damage. Treatment options for COVID-19 are limited and the crude mortality rate estimated by the WHO is around 2.9%. Although a preventive vaccine for COVID-19 could eventually become available, unless sufficient herd immunity is achieved, COVID-19 could potentially cause significant morbidity and mortality over the coming years.
After suffering from an infection, it is common to develop an antibody response against a particular pathogen. Early after infection (usually after the first week), a class of antibodies known as immunoglobulin M (IgM) develops, although these are not typically long-lasting. Later, after the first 2-4 weeks following infection, IgG, a more durable antibody, is produced.
Studies have found that RBD-targeted antibodies are excellent markers of previous and recent infection, that differential isotype measurements can help distinguish between recent and older infections. The detection of IgM and IgG antibodies against SARS-CoV-2 has potential significance for evaluating the severity and prognosis of COVID-19, and even increasing the detection accuracy of nuclear acid test.
The detection of SARS-CoV-2 IgM and IgG is very important to determine the course of COVID-19. Nucleic acid detection combined with serum antibody of SARS-CoV-2 may be the best laboratory indicator for the diagnosis of SARS-CoV-2 infection and the phrase and predication for prognosis of COVID-19.


Test process

Order Information
|
Model |
Description |
Product code |
|
VMGLFA-01 |
20 test/kit, cassette format |
CoVMGLFA-01 |
Product detail pictures:
Related Product Guide:
Assume full responsibility to satisfy all needs of our clients; achieve continual advancements by endorsing the expansion of our purchasers; turn into the final permanent cooperative partner of clientele and maximize the interests of clients for New Arrival China SARS-CoV-2 RT-PCR - COVID-19 IgM/IgG Lateral Flow Assay – Genobio , The product will supply to all over the world, such as: New Zealand, Mombasa, Nepal, We have now a large share in global market. Our company has strong economic strength and offers excellent sale service. Now we have established faith, friendly, harmonious business relationship with customers in different countries. , such as Indonesia, Myanmar, Indi and other Southeast Asian countries and European, African and Latin American countries.
The customer service reprersentative explained very detailed, service attitude is very good, reply is very timely and comprehensive, a happy communication! We hope to have a opportunity to cooperate.