Aspergillus Galactomannan ELISA Detection Kit
Product Introduction
FungiXpert® Aspergillus Galactomannan ELISA Detection Kit is an Enzyme-linked immunosorbent assay for the qualitative detection of Aspergillus galactomannan antigen in adult and pediatric serum samples and bronchoalveolar lavage (BAL) fluid samples.
The incidence of Invasive Aspergillosis (IA) in immunosuppressed patients is rapidly increasing because of antibiotic abuse. IA has a high mortality rate due to lack of typical clinical manifestations and effective early diagnosis methods. Aspergillus fumigatus is one of the most common pathogens that cause severe aspergillus infection in patients with immunosuppressive disease, followed by Aspergillus flavus, Aspergillus niger and Aspergillus terreus.
Characteristics
|
Name |
Aspergillus Galactomannan ELISA Detection Kit |
|
Method |
ELISA |
|
Sample type |
Serum, BAL fluid |
|
Specification |
96 tests/kit |
|
Detection time |
2 h |
|
Detection objects |
Aspergillus spp. |
|
Stability |
The kit is stable for 1 year at 2-8°C |
|
Low detection limit |
0.5 ng/mL |
Background
Invasive Aspergillosis (IA)
Who are susceptible
Patients with prolonged neutropenia, following transplantation or in conjunction with aggressive immunosuppressive regimens.
High incidence
5% to 20%, depending on the patient population.
High mortality rate
50% to 80% due in part to the rapid progression of the infection (ie, 1-2 weeks from onset to death).
Difficult to diagnose
Hard to get histopathological evidence. Sensitivity of culture is low. ≈30% of cases remain undiagnosed and untreated at death.
Galactomannan (GM) test
- An aspergillus specific antigen found in the cell wall that is released during the growth phase of invasive aspergillosis.
- 7 to 14 days before other diagnostic clues become apparent.
Principle
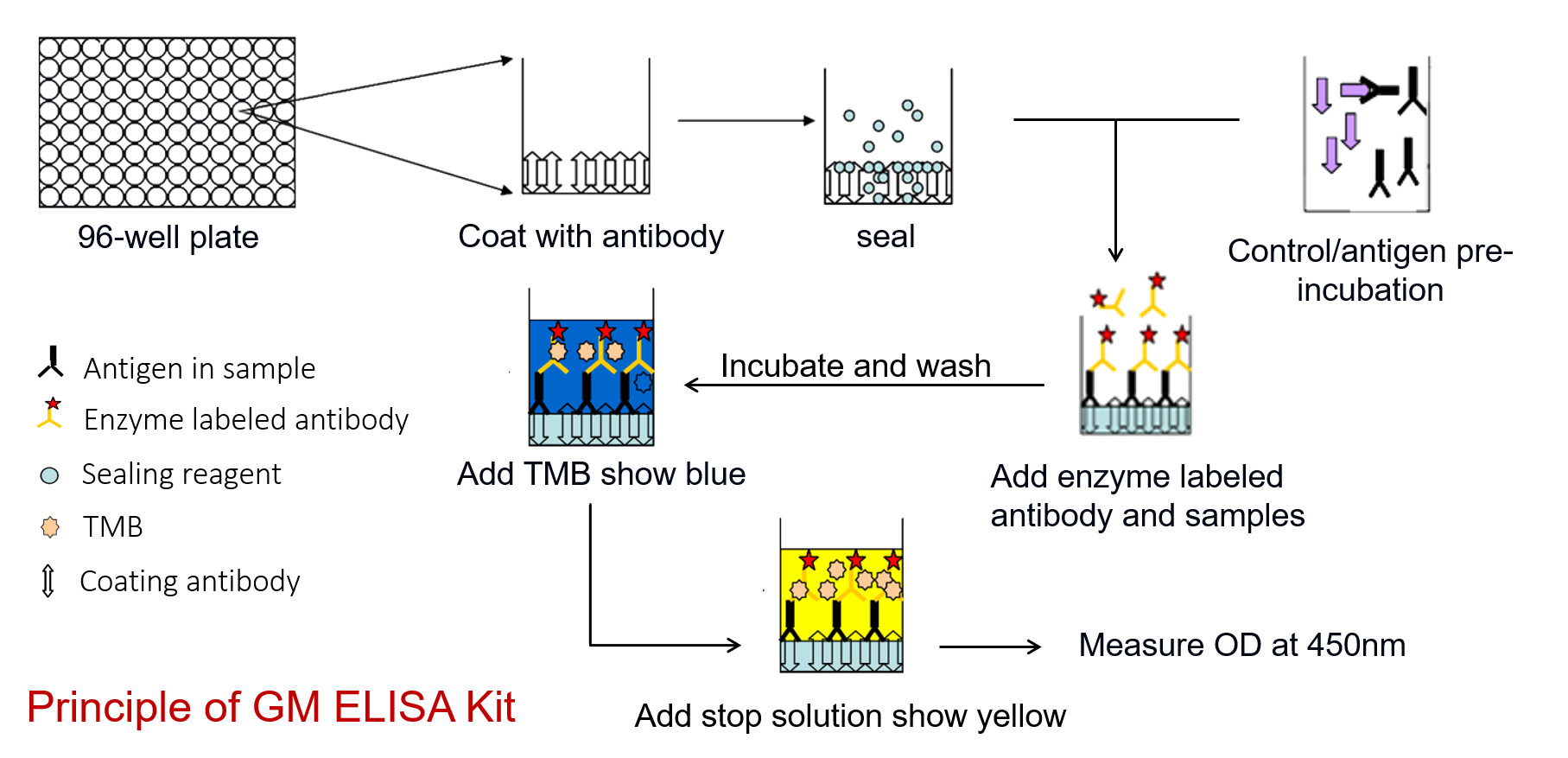
Advantages
- More Advance
International leading edge detection method, high sensitivity and specificity - More Accurate
Optimize the operation process. Reduce the risk of contamination during the experiment - Faster
One-step detection, reducing the number of incubation and washing time
- More Economical
Split microplate, saving cost - Recommendations
Recommended by IDSA guideline for Aspergillosis 2016 and ESCMID-ECMM-ERS guideline for Aspergillosis 2018
Clinical implication
Early diagnosis
- GM is 5-8 days earlier than clinical symptoms of invasive aspergillosis (IA);
- GM is 7.2 days earlier than high resolution CT scan;
- GM is 12.5 days earlier than the start of empirical antifungal therapy.
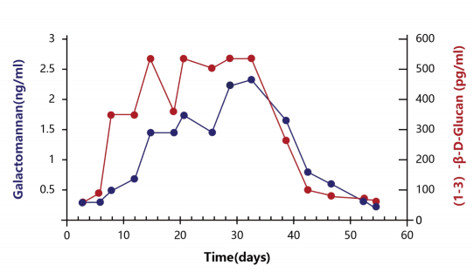
Dynamic monitoring
- GM is proportional to the amount of fungus, which can reflect the infection degree.
- The content of GM antigen decreased with the application of antifungal drugs.
Important medical basis
- Reduce the use of empirical antifungal treatment.
- Strong correlation between outcome and GM index for hematological cancer.
United detection of G and GM test
- Higher specificity and positive predictive value
- Higher sensitivity
Order Information
|
Model |
Description |
Product code |
|
GMKT-01 |
96 tests/kit |
FGM096-001 |