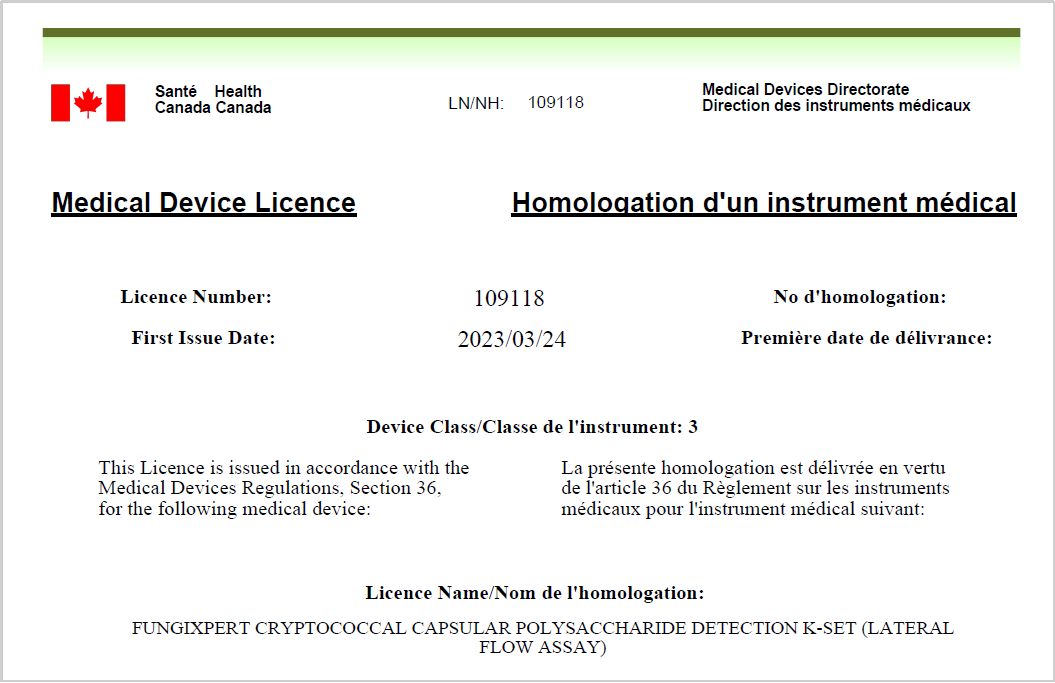
FungiXpert Cryptococcal Antigen Rapid Test Successfully Approved by Health Canada
We are pleased to announce that our Cryptococcal Capsular Polysaccharide Detection K-Set (Lateral Flow Assay) has been granted a registration certificate by Health Canada on March 24th. The assay detects cryptococcal capsular polysaccharides in serum and cerebrospinal fluid, providing a valuable diagnostic for cryptococcal infection.
Cryptococcal infections are caused by the fungus Cryptococcus neoformans, which can be found in soil, bird droppings, and other environmental sources. Infection can occur through inhalation of spores, leading to lung infection or dissemination to other organs, particularly the central nervous system. Cryptococcal meningitis is a major cause of mortality and morbidity in people living with HIV/AIDS in resource-limited settings.
Cryptococcal Capsular Polysaccharide Detection K-Set (Lateral Flow Assay) provides rapid and reliable detection of cryptococcal capsular polysaccharides, enabling early diagnosis and treatment of the infection. The assay is easy-to-use, accurate, and delivers results in just 10 minutes, making it a valuable tool for point-of-care testing in resource-limited settings.
We believe that the Health Canada registration of our Cryptococcal Capsular Polysaccharide Detection K-Set (Lateral Flow Assay) will help to improve the diagnosis and management of cryptococcal infection, and we remain committed to developing innovative solutions to address public health challenges.
For more information on our product, please visit our website or contact us directly.
Post time: Mar-29-2023
